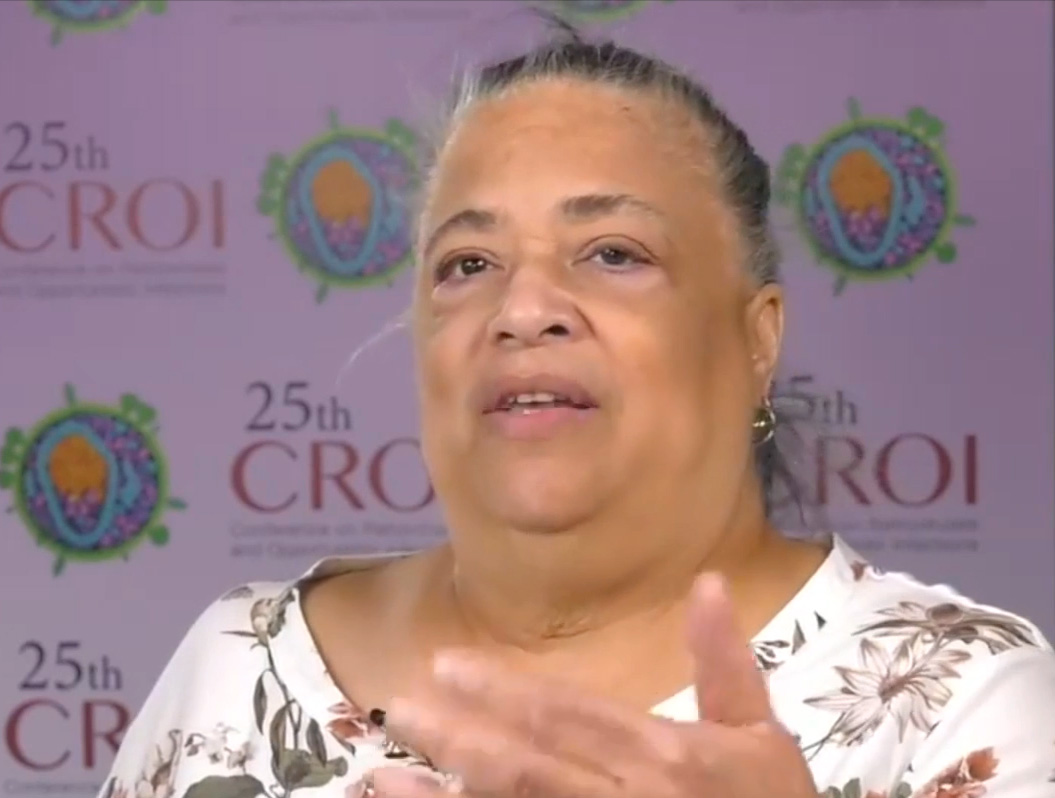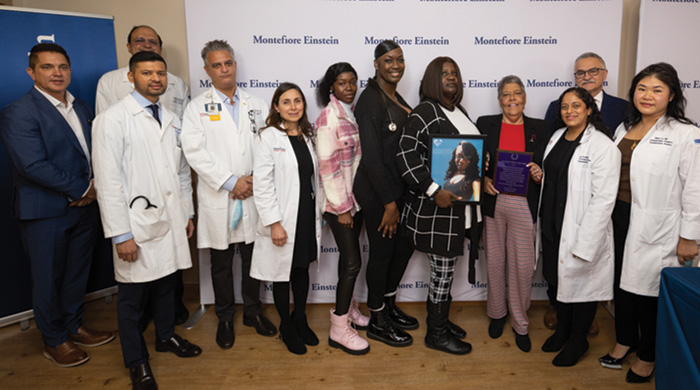There’s a new HIV medication on the market, and it is taken just twice a year. It must, however, be taken with other HIV medications already available. Although most of those are taken daily, they are generally highly tolerable.
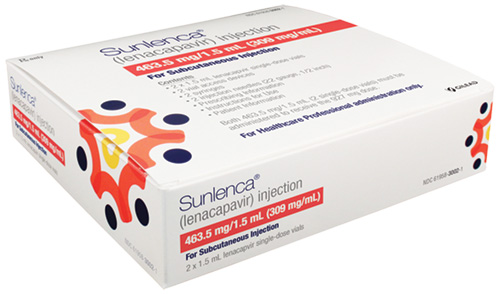
The U.S. Food and Drug Administration (FDA) in December approved Sunlenca (generic name lenacapavir) tablets and subcutaneous injection. Sunlenca is approved for people living with HIV who are heavily treatment-experienced (HTE) with multi-drug resistant (MDR) virus. Their current regimen must be failing due to drug resistance, intolerance (side effects), or safety issues. New and improved options for HIV treatment are always welcome, but especially for this group, who may need the extra help.
Sunlenca is the first in its drug class to be FDA approved. It is a long-acting capsid assembly inhibitor (CAI). Capsid inhibitors work by inhibiting multiple steps of the HIV lifecycle. The Positively Aware 2022 HIV Drug Guide reports that, “Ultimately, it prevents viral RNA from entering the nucleus of human CD4 T cells, halting viral assembly and protein formation and inhibiting assembly of new viral particles. Lenacapavir is highly potent at low doses. Drug efficacy was similar across demographic groups (race, sex at birth, age and geographic region), CD4 cell count and viral load at study entry and which background HIV medications were used.”
FDA approval was supported by one-year data from the Phase 2/3 CAPELLA study. Although its time was limited and the number of participants small, the study showed that these individuals had great success with lenacapavir. Of the individuals randomized to receive lenacapavir, 83% (30 out of 36) achieved undetectable viral load (less than 50 copies/mL). Moreover, they had an average T cell increase of 82. These early results from the ongoing trial were reported at last year’s Conference on Retroviruses and Opportunistic Infections (Virtual CROI 2022). The CAPELLA participants had previously taken a median of nine antiretroviral medications, meaning that half of them had taken more than that.
“Despite the significant advances in ARV [antiretroviral] therapy, there remain numerous critical and pressing unmet needs for people living with HIV,” said Sunlenca’s manufacturer, Gilead Sciences, in a press statement. “This is particularly true for individuals who are heavily treatment experienced—which account for an estimated 2% of adults living with HIV who are on treatment globally—and are unable to maintain virologic suppression due to [drug] resistance, intolerance, or safety considerations. This type of complexity further increases the chance of treatment failure, underscoring the need for new treatment options that are active against resistant variants of the virus with a novel mechanism of action.”
Gilead said lenacapavir is being developed as a “foundation” for several long-acting treatment options in the future. It’s also being studied for HIV prevention (as PrEP).
Sunlenca was approved in the United Kingdom and the European Union last August and in Canada in November. It was expected to be approved in the United States last year, but was held back by safety concerns over borosilicate glass vials used in research. There were no safety concerns with lenacapavir itself.
IAS-USA updates its HIV treatment guidelines
The International AIDS Society-USA (IAS-USA) observed World AIDS Day by issuing updated HIV treatment guidelines. The recommendations include when to start (on the day of or within seven days of diagnosis) and when to switch HIV therapy, including going on the complete regimen taken as two shots every other month (Cabenuva). Also included is information about aging, such as the fact that people starting HIV treatment at an older age experience a weaker response to therapy. Other topics addressed are weight gain, HIV prevention medication (PrEP) and pregnancy. The document points out that cardiovascular disease has decreased in the general population, but not so for people living with HIV.
“As treatment and prevention of HIV improve, new challenges and opportunities arise,” IAS-USA reported in the guidelines. “As people with HIV live longer, there are important considerations related to aging that require an integrated approach. Multidisciplinary and holistic care of people with substance use and substance use disorder is required to achieve optimal outcomes in treating and preventing HIV. Other infectious disease outbreaks, such as COVID-19 and now [mpox] virus infection, also present rapidly evolving challenges for clinicians and people with HIV. To effectively address these and other challenges, as well as to realize the opportunity to end the HIV epidemic, efforts must be redoubled, with equity being the guiding principle.”
Although similar in many ways to recommendations in the HIV treatment guidelines from the U.S. Department of Health and Human Services (DHHS), the IAS-USA guidance differs in many ways as well. For example, it recommends PrEP on-demand scheduling, while DHHS does not. GO TO clinicalinfo.hiv.gov.
The guidelines were published online December 1 in JAMA and are available free of charge. FIND them at iasusa.org.
Dr. Dawn Smith: Advocacy that lives on
The world lost an HIV prevention champion in November with the death of Dawn K. Smith, MD, MS, MPH. Dr. Smith, who was 73, had a long career as an epidemiologist and researcher in biomedical interventions at the Centers for Disease Control and Prevention (CDC). She focused on pre-exposure prophylaxis (PrEP) and worked to help communities understand their need for this intervention to prevent HIV.
Greg Millet, MPH, vice-president and director of public policy at amfAR, was mentored by Dr. Smith when he was a young scientist at the CDC, and has composed a short video of her advocacy at international HIV conferences. It shows, he said, Dr. Smith’s “fierce commitment to HIV prevention and PrEP access for women and people of color.” Writes Millet, “Let’s renew our commitment to ending HIV this World AIDS Day as part of Dawn’s legacy and the legacy of many other leaders that we have lost.”
“For people who would stand to benefit from PrEP, we know that not enough of them even know such a thing exists,” says Dr. Smith in the video. “If patients don’t know that there’s a pill [and now long-acting injectable medication] that prevents HIV, then they’re not going to ask their doctors about it. And as you may know, African Americans are about 14% of the U.S. population. So it was very sobering to see that the proportion of the population that needs PrEP is so heavily weighted towards African Americans and Hispanics.”
In another segment, Dr. Smith addresses medical mistrust: “It is true that there are some historical barriers that keep people, specifically African Americans, from accessing health care. I think the bigger issue for African Americans who are unemployed or who are poor—who are the working poor—is that the cost of health care is the barrier for coming into care for getting services. You may want to put off any kind of health care visit for as long as possible, just for financial reasons. Most members of the Black community understand that hypertension is more common in our population, and they know that they need to get their blood pressure checked. But they don’t understand about HIV. They think it’s just MSM [men who have sex with men]. And if I’m not an MSM, then I don’t have to worry about it. So I think that’s one reason for the disparity, particularly for women.”
She pointed to the need for advocacy groups to be strengthened “so that we can get the message into the community in a different way.”
Get your Misinformation
The Harvard Kennedy School publishes the HKS Misinformation Review, for free to all. In a world filled with distrust of science and “alternative facts,” HIV can kill people who would otherwise live if they turned to accurate, reliable information instead. The review covers various types of lies—scams, disinformation campaigns and conspiracy theories—and the people who propagate them. See how it’s done. GO TO misinforeview.hks.harvard.edu/.
‘HIV and the Journey Toward Zero’
A new documentary film produced by the Chicago Department of Public Health (CDPH) profiles the lives of long-term survivors of HIV as they consider the prospect of ending the epidemic. CDPH has released a trailer for HIV and the Journey Toward Zero, directed by Black queer filmmaker Chan C. Smith. “The ‘end of the HIV epidemic’ is possible in our lifetime,” CDPH says. “It’s an incredible achievement. But as our community celebrates this success, we also need to grapple with the implications. What does ‘the end’ mean for future generations and long-term survivors? How do we address social issues—housing, employment, safety, to name a few—that affect priority communities?” Among the local advocates appearing in the film are Caprice Carthan, Sanford Gaylord, Evany Turk and former Positively Aware editor Jeff Berry, now executive director of The Reunion Project. The film is scheduled for release in February. Watch the trailer at journeytowardzero.com.
Trans-led center receives 340B funding
Arianna’s Center in Fort Lauderdale, which serves a large immigrant base, has become the first trans-led organization in Florida to receive 340B funding. The pharmacy-support program is set to start in early 2023. “We’re helping to lead the way for other trans-led organizations,” said founder and director Arianna Lint. The center will help its clients receive PEP and PrEP as well as hormones in addition to HIV medication. (See Yes, undocumented immigrants can get free PrEP.) Go to ariannas-center.org.
Transplanting hope
It was last spring when Miriam Nieves, 62, a woman in New York state who is living with HIV, received a heart transplant from a young woman in Louisiana who had also lived with the virus. The surgery, which took place at Montefiore Health System near to where Nieves resides, was the first HIV-positive-to-HIV-positive heart transplant in the world. In November, just before Thanksgiving, Nieves met the family of Brittany Newton. Newton, a certified nursing assistant who worked with the elderly, was just 30 when she died. Newton’s mother and two sisters traveled to Montefiore to meet Nieves.
Miriam Nieves (fourth from right) with medical staff and the donor family.
Nieves expressed her overwhelming gratitude, as did her son Edward and her husband of nearly 30 years, Julio. She noted that many people may not even realize that a positive-to-positive transplant can be done, although the HIV Organ Policy Equity (HOPE) Act passed in 2013 makes it possible. Nieves said she hopes to encourage people living with HIV to become organ donors.
Omar Saeed, MD, MS, Nieves’s cardiologist at Montefiore and an associate professor of medicine at the Albert Einstein College of Medicine, with which Montefiore is associated, stressed the point. “By successfully managing Ms. Nieves’s care, we have shown that HIV-positive donors are a lifeline, and we encourage more people to consider sharing this lifesaving gift,” he said.
Montefiore serves residents of the Bronx, along with Westchester County and the Hudson Valley north of New York City.
Newton’s family listened to her heart in Nieves’s chest through a stethoscope. They said their daughter and sister was kind and loving. They said she had a heart of gold.
Montefiore’s press statement is at bit.ly/hiv-heart-transplant.
Help with HIV aging
An online education platform, PRIME Education, has produced the Practical Toolkit for Providing Holistic Care for People Living with HIV, in partnership with The Reunion Project, a national advocacy group for long-term survivors and people aging with HIV. While the toolkit is primarily geared for caregivers and service providers, anyone can view the sessions, which cover:
- optimizing treatment
- comorbidities and co-infections
- assessing and supporting mental, emotional and social wellness, and
- Aging Well with HIV: Taking Ownership of Your Health
The learning modules are available in five languages: English, Spanish, German, French and Italian. Go to bit.ly/TRP-toolkit.
Walmart to open new HIV specialty pharmacies
Walmart has announced expansion of its HIV specialty pharmacies, with four new pharmacies set to open at store locations in New York, New Jersey and Connecticut within the first three months of this year.
The expansion builds on a pilot program launched in 2021 in which three Special Pharmacies of the Community (SPOC) were opened in Florida and Georgia. The specialty pharmacies are located within already existing pharmacies at Walmart stores that include specialized HIV-trained pharmacists, enhanced coordinated care and emotional support services.
The company is also partnering with the Elton John AIDS Foundation to offer a nationwide HIV Prevention and Treatment Continuing Pharmacist and Provider Training Program. Designed by Duke University, the program has been beta tested by pharmacists at the four new locations and by all of Walmart Health’s community health workers.
“For patients with HIV and populations at greatest risk, Walmart can play an important role in understanding each individual and the unique challenges they face, and our pharmacists play an important role in the treatment paradigm, especially for patients with HIV who may be on a complex medication regimen,” said Dr. John Wigneswaran, Walmart’s chief medical officer.
Expected to open in the first quarter of 2023, the new specialty pharmacies will open at these store locations:
- 979 Route 1
North Brunswick, NJ
- 41 Anawana Lake Rd.
Monticello, NY
- 2100 88th St.
North Bergen, NJ
- 495 Flatbush Ave.
Hartford, CT
Based in Bentonville, Arkansas, Walmart made the announcement coinciding with World AIDS Day in December. Other retail pharmacies, such as CVS and Walgreens, also operate HIV specialty pharmacies.
—Rick Guasco
DHHS now supports breastmilk feeding by parents living with HIV
In its updated guidelines for infant feeding, the U.S. Department of Health and Human Services (DHHS) has come out in support of parents’ shared decision making with care providers in clarifying its guidance for birthing parents who are living with HIV.
The updates also state that “it is inappropriate to engage Child Protective Services (CPS) or similar services in response to infant feeding choices” of parents living with HIV.
“To see that the voices of those of us living with HIV have been able to invoke change on this level is inspiring,” said Ciarra “CiCi” Covin, program manager at The Well Project and a woman living with HIV who breastfed her most recent child. “It now feels as if we are one step closer to decriminalizing breast/chestfeeding among parents living with HIV since the guidelines specifically recommend against calling CPS. From my own personal experience, I know that this threat is one of the scariest, and by removing it, pregnant people living with HIV will have more confidence to exert agency over their bodies and how they care for their families.”
“People with HIV [PLWH] who are considering conception, are pregnant or in the postpartum period should receive evidence-based counseling to support decision making about infant feeding,” said DHHS in a statement. The updated guidelines note that:
- Women and other parents living with HIV should receive evidence-based, patient-centered counseling to support shared decision-making about infant feeding
- Counseling about infant feeding should begin prior to conception or as early as possible in pregnancy and should communicate the fact that achieving and maintaining viral suppression through antiretroviral therapy during pregnancy and postpartum decreases the risk of breastfeeding transmission to less than 1 percent
Providers should support parents’ infant-feeding choices, the updates advise, including:
- Women and other birthing parents living with HIV who have a sustained undetectable viral load while on ART who choose to breastfeed
- Women and other birthing parents living with HIV who choose to formula feed (providers should
ask about potential barriers to formula feeding and explore ways to address them)
The new guidance appears in DHHS’ Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States, also known as the Perinatal HIV Clinical Guidelines.
The updates were issued by the DHHS Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission in the U.S., a group under the NIH led by academics and experts comprised of about 40 members with lived experience and expertise in caring for pregnant persons living with HIV.
The panel included community input in the development and revision process of the infant feeding guidelines, and specifically reached out to community partners to gather feedback. In collaboration with the DHHS Pediatric Antiretroviral Panel, the Perinatal Panel aimed to balance the low risk of transmission with strong community desire to breastfeed, acknowledging community concerns and the desire for bodily autonomy, while promoting a greater focus on shared decision making.
“These updates emphasize the nuanced nature of infant-feeding decisions, the need to honor parents’ bodily autonomy, and the important role providers play in supporting women and other parents living with HIV in their decision-making processes,” said Krista Martel, executive director of The Well Project.
The revised guidelines also make note of the term “breastfeeding,” which is used to describe feeding a child one’s own milk, either direct feeding or expressed milk, acknowledging that gender-diverse individuals and transgender men might prefer the word “chestfeeding.” “When counseling individuals with HIV about infant feeding, it is important to assess and use their preferred terminology,” the update advises. “We urge providers to consult community-based resources for more information about inclusive, affirming language around gender in health care settings.”
Read the updated guidelines at clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new.
Mosaico HIV vaccine trial fizzles
A major Phase 3 clinical trial of an HIV vaccine has been halted because it was found to be no more effective than a placebo at preventing HIV.
The Mosaico study (also known as HPX3002/HVTN 706) involved 3,900 cisgender men and transgender participants who have sex with cisgender men and/or transgender people in Argentina, Brazil, Italy, Mexico, Peru, Poland, Puerto Rico, Spain and the United States.
The vaccine was a “mosaic” of multiple HIV subtypes (hence the study’s name) in the hopes of prompting a person’s immune system to produce antibodies resistant to a variety of HIV strains predominant around the world.
During a regularly scheduled review of the study, an independent data and safety monitoring board, however, found that while the Mosaico vaccine was safe, it did not offer any more protection from HIV than the placebo arm of the trial. During the clinical trial, all participants were offered comprehensive HIV prevention tools, including pre-exposure prophylaxis (PrEP). Participants who acquired HIV during the trial were promptly referred to medical care and treatment.
The news was not unexpected. A study of a similar vaccine conducted in sub-Saharan Africa among young women—a Phase 2b trial known as Imbokodo (HPX2008/HVTN 705)—was ended in August 2021 after a data review determined the vaccine was also safe but ineffective against acquiring HIV.
Mosaico and Imbokodo were the only vaccine studies that were this far along in testing. No other vaccine candidates are expected to reach a similar stage until at least 2024, clinicians expect.
“HIV is a constantly changing and very challenging adversary,” said Larry Corey, MD, one of the study’s principal investigators at HVTN at the Fred Hutchinson Cancer Center in Seattle. “We can become disappointed when our best efforts don’t produce the results we’re looking for.”
Still, Mosaico has been praised for its inclusion of diverse populations affected by HIV, setting a standard for future vaccine studies.
“It was important to all of us that diverse communities were engaged early on, and often, in the design phase and throughout the life of the study,” said Stephaun Wallace, PhD, MS, a research epidemiologist and principal staff scientist at Fred Hutch who directed community engagement for the study globally. “From the various symposia, community and ethics consultations, and study meetings where community representatives on the study team were present, we received great feedback and perspective that informed how we collectively moved this study from concept to implementation.”
Mosaico was led by a global public-private partnership including Janssen Vaccines & Prevention B.V., the National Institute of Allergy and Infectious Diseases (NIAID), HVTN and the U.S. Army Medical Research and Development Command.
—Rick Guasco
World AIDS Day 2022: A global look at HIV today
World AIDS Day, December 1, offers the opportunity to take stock of the global epidemic. Organizations and government bodies review the numbers, examine the progress and assess what the future may bring. The theme for the 2022 commemoration was “Putting Ourselves to the Test: Achieving Equity to End HIV,” backed by the slogan, “Equalize.” According to UNAIDS, “The inequalities which perpetuate the AIDS pandemic are not inevitable; we can tackle them… UNAIDS is urging each of us to address the inequalities which are holding back progress in ending AIDS. The ‘Equalize’ slogan is a call to action.” Here’s a roundup of World AIDS Day announcements that serves as a starting point for the global state of HIV for the year ahead.
U.S. progress on HIV/AIDS
For World AIDS Day 2022, the White House issued an extensive report on the administration’s efforts to end the epidemic at home and abroad. The priorities of the Biden-Harris administration include:
- affirming U=U (“undetectable equals untransmittable,” meaning that people on antiretroviral treatment with a viral load less than 200 cannot pass the infection to their sex partners)
- proposing a 10-year, $9.8 billion budget for creating a National PrEP Program to “guarantee PrEP at no cost for all uninsured and underinsured individuals”
- modernizing HIV-specific criminal laws, which includes promoting an understanding of science in the legal system
- strengthening protections against discrimination based on HIV status and
- addressing violence against transgender women of color and promoting gender-affirming care.
Details of the Biden plan are at bit.ly/Biden-HIV-efforts.
WHO urges equity
In its statement, the World Health Organization said, “WHO is calling on global leaders and citizens to boldly recognize and address the inequalities which are holding back progress in ending aids; and equalize access to essential HIV services particularly for children and key populations and their partners—men who have sex with men, transgender people, people who use drugs, sex workers, and people in prisons.”
UNAIDS calls inequality ‘dangerous’
One day ahead of World AIDS Day, UNAIDS declared that differences in access to HIV prevention and care is stopping the world from ending AIDS.
The report Dangerous Inequalities gives data and advocacy in three specific areas of concern:
- gender-based differences that include men not testing for the virus and getting it treated;
- stigma and discrimination against “key populations” that also prevent access to health care, such as criminalization of gay sex; and
- the gaps in treatment and services between children and adults.
According to the report, “Stigma and discrimination have profound negative impacts on the health and well-being of key populations and on the success of AIDS responses for these groups. For instance, a meta-analysis across five of 10 reviewed studies found that people living with HIV who perceive high levels of stigma and discrimination are 2.4 times more likely to delay enrollment in care until they are very ill. A global review comparing national law and policy frameworks with HIV outcomes also found that knowledge of HIV status and rates of viral suppression were lower in countries that criminalized same-sex sexual acts or drug use, with outcomes that were 18–24% worse in countries that criminalized both in addition to sex work.”
Inspired by the “Equalize” motto, the report calls for “equal access to rights, equal access to services, equal access to science and equal access to resources.”
“We can end epidemics among people who inject drugs, but we are failing to do so because we are not ensuring meaningful access to harm reduction services,” declares one heading. The section goes on to say that, “We have the means to prevent HIV transmission among people who inject drugs. A number of high-income countries have virtually eliminated new HIV infections among people who inject drugs by implementing a comprehensive package of harm reduction services, including needle and syringe programs, opioid agonist maintenance therapy, and HIV and primary health-care services.”
“Leaving inequalities to fester is perpetuating the AIDS pandemic, endangering everyone,” writes UNAIDS executive director Winnie Byanyima in the foreword. “Tackling inequalities will not only help the marginalized, it will help everyone.”
Read and download a PDF of the report at bit.ly/UNAIDS-Equalize-report.
“This could be the year that marks a fundamental reshaping and reimagination of HIV prevention, if everyone does their part,” says the HIV prevention advocacy group AVAC. There are now multiple options for preventing HIV—one injection every two months (Apretude), two different daily oral medications that confer more than 99% protection against the virus (Descovy and Truvada for PrEP), and a vaginal ring with antiretroviral medication that is being approved in other countries. AVAC called for this menu of prevention products to be offered “at a scale that will reach everyone who needs prevention.” The organization noted that, “Global leaders, confronting heartbreaking rates of HIV infection that have been stalled at about 1.5 million new diagnoses a year for several years, are calling for bold new initiatives.” AVAC also quoted Anthony Fauci, MD, who retired in December as director of the National Institute of Allergy and Infectious Diseases (NIAID), from its podcast that week: “We’ve got to get user-friendly PrEP to [people]. That’s going to involve implementation that integrates itself into the health care delivery system. Otherwise, we’re going to have highly effective interventions that are not being maximally utilized, and that would really be a big mistake.” Details are at avac.org.
NIH reviews treatment, a vaccine and the cure
Dr. Fauci and Maureen M. Goodenow, PhD, director for the Office of AIDS Research at the National Institutes of Health (NIH), issued an overview of HIV treatment, vaccine development and cure efforts from the institutes.
“Innovative research led to the highly effective HIV treatment and prevention modalities available today,” they wrote. “However, each year new HIV infections occur at an unacceptably high rate. In 2021, roughly 1.5 million people worldwide, including 160,000 children, were newly infected with HIV, and an estimated 650,000 people died, according to the Joint United Nations Programme on HIV/AIDS (UNAIDS). In the U.S., approximately 35,000 new HIV cases are diagnosed each year. Stigma, racism, housing and food insecurity, substance use disorder, and other socio-structural factors serve to place the heaviest HIV burden on marginalized and minority populations, including men who have sex with men, transgender women, people who use drugs, and members of the Black and Hispanic/Latino communities. This year’s World AIDS Day theme, ‘Putting Ourselves to the Test: Achieving Equity to End HIV,’ ties into one of the cornerstones of the National HIV/AIDS Strategy (NHAS)—addressing disparities and health inequities to ensure that HIV prevention and treatment services reach the people who need them most. NIH research plays a major role in the plan’s goal of reducing new HIV cases in the United States by 75% in 2025 and by 90% in 2030.”
As is often the case, progress does not proceed smoothly: “The global scale-up of life-saving antiretroviral treatments (ART) has led to a 40% reduction in HIV/AIDS mortality since 2010 and, subsequently, has helped reduce HIV transmission. However, too many people who should be on ART are not. Specifically, as of the end of 2021, there were 38.4 million people currently living with HIV and 28.7 million people on ART—a treatment gap of 9.7 million people. Antiretrovirals not only improve and maintain the health of the individual with HIV, but also prevent transmission to others. Global promotion of the ‘undetectable equals untransmissible,’ or U=U, benefit of ART has helped raise awareness, but more must be done to ensure that people diagnosed with HIV have access to treatment—as early as possible in their infection—and remain in care. Long-acting ART (cabotegravir plus rilpivirine) delivered via monthly [or every other month] injection offers an alternative to a life-long regimen of daily oral ART that may be preferable for many people. Injectable cabotegravir received FDA approval late last year for pre-exposure prophylaxis (PrEP) based on data from the NIAID-sponsored HPTN 083 and HPTN 084 studies. These clinical trials found that injectable cabotegravir—given as infrequently as six times per year—was more effective at protecting against HIV acquisition than oral ART. Follow-on data from those studies found the protective effect was sustained for as long as 12 months. Hopefully, this approach to protect against HIV infection will be made available to many more people, as oral PrEP has been generally underutilized.”
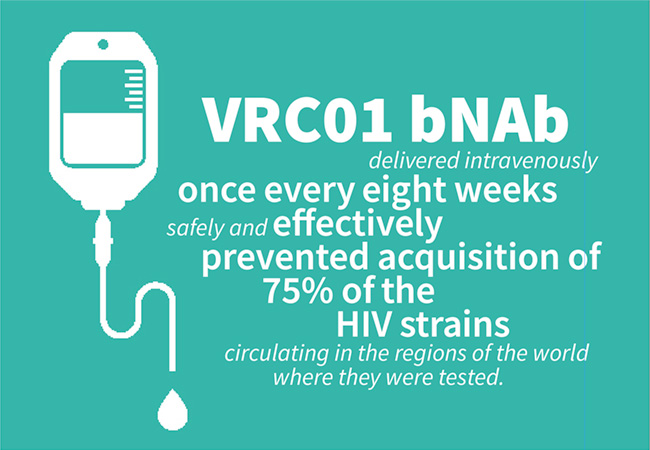
Not just treatment and prevention, but an innovative way to take medication—and using less of it—has moved forward: “Broadly neutralizing antibodies (bNAbs) that can neutralize multiple genetically distinct HIV strains represent a potential new strategy for HIV treatment and prevention,” Fauci and Goodenow reported. “[In 2021], the NIAID-developed VRC01 bNAb delivered intravenously once every eight weeks safely and effectively prevented acquisition of 75% of the HIV strains circulating in the regions of the world where they were tested. This year, a small study conducted by NIAID scientists and their collaborators found that people who began ART during the early stages of their HIV infection achieved a long period of HIV suppression (nearly 40 weeks) without ART after receiving an infusion of two investigational bNAbs. As next-generation antibodies with increased potency and durability are developed, there is hope that infrequently administered bNAbs, possibly with a long-acting injectable ART, could lead to HIV suppression for years, not months.
Building on lessons learned from the COVID-19 vaccines, the NIH recently launched the Phase 1 HVTN 302 trial evaluating three investigational HIV vaccine candidates based on a messenger RNA (mRNA) platform.”
Last but not least, they look at a cure. “On the HIV cure front, the third and fourth documented cases of HIV remission in the absence of ART following a stem cell transplant were made public. Additionally, researchers at NIAID’s Vaccine Research Center and their collaborators developed a cutting-edge technology that revealed new insights into HIV cellular reservoirs. This research could lead to new strategies for targeting latent virus. HIV therapies involving the CRISPR gene editing tool also entered early-stage testing in 2022.”
Hepatitis was also important: “There was some encouraging news for people with HIV who had never been vaccinated against or infected with hepatitis B virus (HBV). People living with HIV, including those on ART, are at greater risk of liver-related illness and death when co-infected with HBV. The ongoing, NIAID-sponsored ACTG A5379 study found that a three-dose course of the HEPLISAV-B hepatitis B vaccine resulted in significant levels of protection for study participants.”
Promoting prevention
“This could be the year that marks a fundamental reshaping and reimagination of HIV prevention, if everyone does their part,” says the HIV prevention advocacy group AVAC. There are now multiple options for preventing HIV—one injection every two months (Apretude), two different daily oral medications that confer more than 99% protection against the virus (Descovy and Truvada for PrEP), and a vaginal ring with antiretroviral medication that is being approved in other countries. AVAC called for this menu of prevention products to be offered “at a scale that will reach everyone who needs prevention.” The organization noted that, “Global leaders, confronting heartbreaking rates of HIV infection that have been stalled at about 1.5 million new diagnoses a year for several years, are calling for bold new initiatives.” AVAC also quoted Anthony Fauci, MD, who retired in December as director of the National Institute of Allergy and Infectious Diseases (NIAID), from its podcast that week: “We’ve got to get user-friendly PrEP to [people]. That’s going to involve implementation that integrates itself into the health care delivery system. Otherwise, we’re going to have highly effective interventions that are not being maximally utilized, and that would really be a big mistake.” Details are at avac.org.