It’s official. Cabenuva, a long-acting regimen given as two injections once every two months, was approved by the U.S. Food and Drug Administration (FDA) in January of last year for maintenance therapy (switching from another regimen, with undetectable viral load). This year, in March, the FDA updated Cabenuva’s drug label, making the recommended oral lead-in optional before starting injections.
“Oral cabotegravir and rilpivirine [the medications comprising the long-acting regimen] can be taken for a month to assess tolerability to the medicines contained in Cabenuva,” said manufacturer ViiV Healthcare in a press release. “However, this oral lead-in is now optional after clinical trial data demonstrated similar safety and efficacy profiles for both initiation methods (with or without the oral lead-in).”
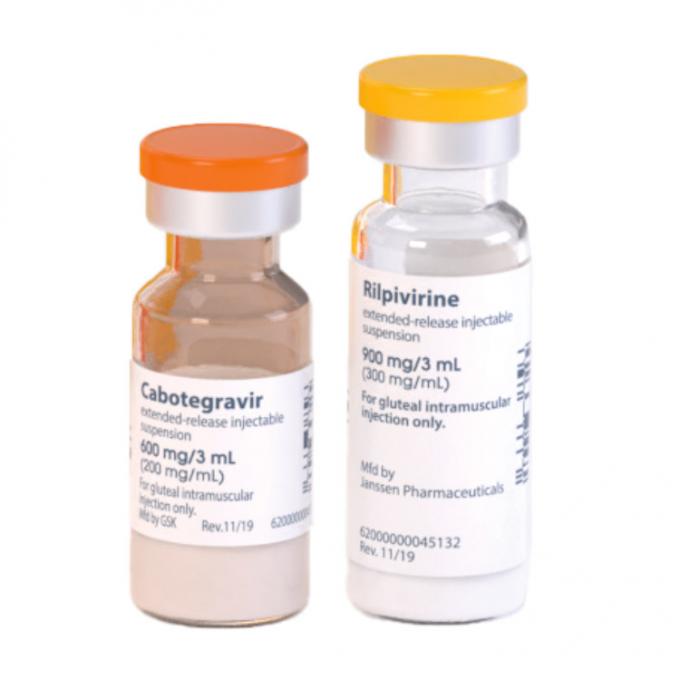
Because the two drugs that make up Cabenuva are administered as long-acting injections, an oral lead-in consisting of the two medications in pill form was recommended in order to first check for side effects before going on the injections. The pills are also used to keep people on their treatment if they are unable to go to the clinic for the injections.
The label change is based on Phase 3 data at 124 weeks from the FLAIR (First Long-Acting Injectable Regimen) study, which showed that whether people went immediately on Cabenuva injections or began with a month of oral lead-in pills, the results were similar for virologic suppression (achieving undetectable viral load), safety, tolerability, and pharmacokinetics (how substances move through the body, including absorption, metabolism, and elimination).
Cabenuva can also be taken once monthly, still with the two injections. Cabotegravir injections by themselves were approved for HIV prevention in December 2021, under the brand name Apretude, given as one injection every other month (go to positivelyaware.com/cabenuva and positivelyaware.com/apretude).
Cabenuva now for adolescents
More good news on Cabenuva: in March, the FDA approved its use for adolescents ages 12 and older who weigh at least 77 pounds (35 kg). As with adults, adolescents must first be on stable HIV therapy with no history of treatment failure and no known or suspected drug resistance to the medications that make up Cabenuva (cabotegravir and rilpivirine).
The approval came based on data in adults and from 16 weeks of analysis in the ongoing MOCHA (More Options for Children and Adolescents) study. MOCHA is conducted in collaboration with the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT).
There were 23 adolescents in the MOCHA analysis. They received either cabotegravir or rilpivirine in addition to the HIV therapy they were already taking. Thirteen experienced injection site pain (all Grade 1 or 2, meaning mild or moderate). Two experienced insomnia (one of which was Grade 3, “severe”). One experienced Grade 3 hypersensitivity (allergic reaction) to rilpivirine that led to discontinuation of the drug during the oral lead-in.
HIV Long-Term Survivors Awareness Day is June 5
Sunday, June 5 is HIV Long-Term Survivors Awareness Day (#HLTSAD). This year’s theme is “Mobilize to Thrive.”
“Today, HIV Long-Term Survivors (HLTS) represent a diverse group of people diagnosed with HIV before the advent of Highly Active Antiretroviral Therapy or HAART in 1996,” says the community-based group Let’s Kick ASS (AIDS Survival Syndrome), which first organized the awareness day. “Often overlooked HLTS include people born with HIV or who acquired the virus as babies and are now in their 30s and 40s. HLTS are also those living with HIV and AIDS for over 25 years. We are developing a working social media campaign and tangible calls to action to improve the quality of our lives.”
Among the priorities of Let’s Kick ASS:
- improving the quality of life for people aging with HIV/AIDS
- demanding universal treatment access to help end the HIV epidemic
- prioritizing culturally aware mental health care
- overcoming the challenges of poverty and economic insecurity
- fighting against “discrimination and invisibility facing older adults with HIV and AIDS” (the group declared that “ageism is a virus”)
Let’s Kick ASS (LKA) calls universal treatment access “the message of Undetectable = Untransmittable” (U=U). The group invites long-term survivors to share their stories, wisdom, and lessons. This in turn can help set an agenda for needed changes. Photos and videos are welcome.
Watch a poignant short video of LKA members speaking to their issues at hltsad.org, where people can also sign up for the group’s HIV Long-Term Survivors League newsletter.
FDA approves first condom for anal sex
The first condom recommended specifically for use in anal sex has been approved by the U.S. Food and Drug Administration (FDA). Manufactured by Global Protection Corp., the ONE Male Condoms has also been approved as a contraceptive and for reducing sexually transmitted infections during vaginal intercourse.
“[T]his authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” said Dr. Courtney Lias, Ph.D., director of the FDA’s Office of Gastrorenal, ObGyn, General Hospital, and Urology Services, in a press statement. She added that the FDA’s go-ahead for the anal condom establishes a regulatory system for other condom brands to seek market approval and increase condom use.

Condoms have long been recommended for use during anal sex, but prior to approval of the One Condom, none had been marketed for anal sex in the U.S. This is due in part to a lack of sufficient data to prove the safety of condoms used for this purpose. Anal sex poses the highest risk of HIV transmission when performed without protection; PrEP and condoms are both forms of protection against HIV.
In the largest study of its kind by Global Protection Corp. and Emory University, ONE Male Condoms had a failure rate of less than 1%, significantly lower than the 5% failure rate set by the FDA. Conducted between May 2016 and May 2017, the study involved 504 men engaging in 2,351 anal and 2,533 vaginal acts of intercourse. The study defined failure as any slippage or breakage of the condom occurring during intercourse.
ONE Male Condoms are available in three versions: thin, standard, and fitted; the fitted version offers 54 different sizes. According to the trial study, the condom version has no effect on its efficacy. —Jack Reding
Cancer and HIV in men
Men living with HIV had nearly twice the rate of cancer as did men who were HIV-negative, according to a research team analyzing Medicaid data.
“Cancer is one of the most common comorbidities in men living with HIV (MLWH),” reported Siran M. Koroukian, PhD, and colleagues in the March 14 issue of Cancer, a journal of the American Cancer Society. “However, little is known about the MLWH subgroups with the highest cancer burden to which cancer prevention efforts should be targeted. Because Medicaid is the most important source of insurance for MLWH, we evaluated the excess cancer prevalence in MLWH on Medicaid relative to their non-HIV counterparts.”
Among the MLWH, cancer was found much more often in those who had symptomatic HIV than those who did not have symptoms of HIV disease.
The cancers most often found in MLWH, whether the men had symptomatic HIV or not, were anal cancer and lymphoma.
The greatest difference found was a higher prevalence of anal cancer in younger men with HIV, whether symptomatic or not. In terms of race/ethnicity, the highest incidence of anal cancer was in Hispanic men.
“Given the Medicaid program’s role in insuring MLWH, the current findings highlight the importance of the program’s efforts to promote healthy behaviors and vaccination against human papillomavirus [HPV] in all children and adolescents and to provide individualized cancer screening for MLWH,” the team said in its conclusion.
According to an accompanying editorial, “People living with HIV have a great incidence of some cancers, and many of these people can [be] expected to live long-term and will require treatment for cancer. Because many of them are also insured by Medicaid, new approaches to public health practice and policy are needed.”
There were 82,495 MLWH in the analysis, compared with more than seven million men who did not have HIV, all between the ages of 18 and 64.
The study was presented in part at the 44th Annual Meeting of the American Society of Preventive Oncology held virtually in March.
HIV vaccine trial begins enrollment—and dosing
The Fred Hutchinson Cancer Research Center announced that, “The first 12 study participants have been enrolled in a new Phase 1 clinical trial using the messenger ribonucleic acid (mRNA) vaccine technology developed by Moderna. The study evaluates the safety of and immune responses to three different experimental vaccines against HIV. This randomized, open-label trial represents one of the first clinical studies of the use of mRNA vaccine technology against HIV.”
Fred Hutch, as the center is also known, is the international headquarters for the HIV Vaccine Trials Network (HPTN). HPTN is a publicly-funded international collaboration.
“The investigational vaccines are not expected to provide protection from HIV infection, yet the knowledge gained from this study will aid in the future development of an HIV vaccine regimen,” the institute continued in its March 14 press release. “Researchers hope to learn whether the immune system will respond to the experimental vaccines by making antibodies and T cells that could fight HIV if a person is ever exposed to the virus in the future. The trial will also build knowledge about how the immune responses to an mRNA vaccine compared to the responses to protein-based vaccines, while helping define the potentials of using mRNA to increase the pace of developing an HIV vaccine.”
Moderna gained fame for its mRNA technology that was successfully used for developing a vaccine against COVID-19.
“The experimental vaccines carry mRNA, a piece of genetic code, delivering instructions to cells for making proteins, in the same way that the mRNA vaccines against COVID-19 instruct the body’s cells to make the SARS-CoV-2 spike protein,” Fred Hutch explained. “These instructions show human muscle cells how to make small portions of proteins that resemble parts of HIV, but are not the actual virus. People cannot get HIV from the vaccines. Once human immune cells have used the instructions, the mRNA is quickly broken down, and does not stay in the body.”
“Developing a vaccine regimen that induces sustained protective levels of HIV neutralizing antibodies in humans has been difficult to achieve. At Moderna, we believe that mRNA offers an opportunity to take a fresh approach to this challenge,” said Stephen Hoge, M.D., president of Moderna, in a company press release.
The company announced that it had dosed its first study participant in the trial.
The HVTN 302 trial is sponsored by the Division of AIDS (DAIDS) of the National Institute of Allergy and Infectious Diseases (NIAID) within the National Institutes of Health (NIH). Up to 108 HIV-negative adults will be enrolled.
Go to the statements of Project ACHIEVE and Columbia University, in New York City, which are also participating in the study: harlemworldmagazine.com/new-york-blood-center-and-columbia-university-in-harlem-conduct-groundbreaking-hiv-vaccine-research-study, and of the NIH at hiv.gov/blog/nih-launches-clinical-trial-three-mrna-hiv-vaccines.
AIDSWatch 2022
Each year during the advocacy event AIDSWatch, people from around the country go to Washington, D.C. to talk with members of Congress and staff about urgent issues related to HIV/AIDS.
Advocates from 34 states held meetings with more than 135 offices on Capitol Hill during this year’s virtual AIDSWatch, according to the organizer of the event, AIDS United. This year’s event focused on:
- supporting the health of people living with and vulnerable to HIV
- addressing structural and institutional racism and other inequities, and
- increasing funding for programs that address the HIV, STD, and overdose epidemics
Martha Cameron, from the US PLHIV Caucus, urged participants to “make sure you talk about quality and gender-affirming care from providers that are well-versed on their patients’ options and rights. Make sure you talk about expanding HIV prevention and coverage of PrEP. Make sure you talk about protecting our rights to vote. Make sure you talk about reforming and repealing HIV criminalization laws. Make sure you talk about sexual and reproductive health.”
Assistant Health Secretary Rachel Levine delivered the keynote address during the annual advocacy event, held in April. She noted President Joe Biden’s update to the National HIV/AIDS Strategy (NHAS), released on World AIDS Day, December 1. Not just federal, but also non-federal, groups are called to “accelerate efforts to end the HIV epidemic in the United States by 2030,” because federal action alone is not enough. “We need all of you, and the organizations, individuals, and programs you represent to also innovate and collaborate in ways aligned with the strategy,” she said. She also promoted the president’s request for a new program to promote HIV prevention via PrEP (pre-exposure prophylaxis), medication that stops infection in its tracks, providing the treatment of uninsured and underinsured individuals.
Said AIDS United’s vice president, Carl Baloney Jr., “We absolutely have the tools necessary to end the HIV epidemic. We have overcome odds that have seemed insurmountable before. Together we can show the world what an HIV advocate can do, and more, what we can do together. Together, we can end the HIV epidemic.”
Go to AIDS United’s YouTube channel for more.
Latino web series updates the narrative of HIV
Once again, the pioneering bilingual media company Latino Alternative TV (LATV) takes aim at stigma and discrimination through the stories of community members living with HIV. The series of online video shorts was birthed by the company’s creative team which focuses on LGBTQ+ advocacy. Living y Ready explores personal issues such as disclosure experiences, self-harm, substance use, and depression, but teams them up with facts, resources, and science.
“We created Living y Ready to update the narrative around HIV in the media because the conversation today needs to be about living with HIV, not just tragedy and loss,” says the creator and director of the series, Andres Palencia. “The people living with HIV featured on the series are talented, ambitious about their future, and making a difference in the world. I have a deep admiration and respect for them and I know a lot of our audience will see hope for themselves by getting to know the cast of Living y Ready.”
The show premiered April 13 and continues through Pride Month, scheduling its last episode on June 22. “Living y Ready was created to have genuine conversations that reframe the narrative around the stigma of HIV through informative segments, candid round table conversations, and queer expression with the same goal as [the company’s video series] My Health Agenda, to continue creating safe spaces for the LGBTQ+ community to be open, honest, and real without the fear of judgement and rejection,” says an LATV press release.
The panelists living with HIV who join the series include Alfredo Trejo, a scholar and artist currently working on his PhD; Erick Velasco, host of “The Homo Homie Podcast;” Mallery Robinson, an Afro-Caribbean transwoman who works as an advocate for transgender and HIV healthcare (and who was among the people featured on the cover of the Positively Aware 2022 HIV Drug Guide); Jennifer Rodriguez, a Latinx transgender woman who is a trans recovery advocate; Melody Torres, National Director of Health and Wellness for FLUX; and Miranda Ramirez, a PrEP navigator helping people gain access to HIV prevention medication.
The experts joining in the conversation are Natalie Sanchez, MPH, Director of UCLA Family AIDS Network; Hilda Sandoval, PhD, LMFT, Director of Behavioral Health Programs at JWCH Institute, Inc.; and Ray Fernandez, AMFT, Patient Care Manager for AltaMed Healthcare. José Ramos, National Director of Sales at the AIDS Healthcare Foundation, will host the informational episodes.
Sanchez and Sandoval previously collaborated on a bilingual telenovela (similar to a soap opera) exploring HIV stigma, Sin Verguenza (see May+June 2018 Positively Aware). Go to latv.com.
Empowering young adults through storytelling
Howard University has launched a new program for young adults (18–29) in the mid-Atlantic region who are living with HIV. StoryTIME: Telling Is My Empowerment uses photography as a starting point to share experiences and break down HIV stigma. The program provides mentorship, leadership, and advocacy skills to help their participants become leaders in their communities.
The program takes place mostly online, consisting of 8–10 virtual sessions over a 12-week period. Two in-person sessions per year take place at Howard University’s Washington, D.C. campus. The program offers transportation assistance for the in-person sessions, and participants are compensated for their time in gift cards. There are two groups in staggered succession. The first group mentors the second in weekly meetings.
StoryTIME is open to young adults living in states from Virginia to New Jersey. To apply, email Patty Houston: phouston@howard.edu.
—Jack Reding
Prison Journalism Project appoints its first board
The Prison Journalism Project, a national initiative that trains incarcerated writers to be journalists and gets their stories published, appointed its first board of directors in March. The seven board members represent several professional disciplines in media and law.

Yukari Kane
“Each of PJP’s newly appointed directors shares our vision of building a strong network of prison journalists and brings a unique array of ideas and experiences to help us continue to grow,” said board co-chair and PJP co-founder Yukari Kane in a press release.
PJP was recently awarded a major grant from the FWD.us Education Fund to expand its newsroom operations and its journalism school program.
Go to prisonjournalismproject.org. Write to Prison Journalism Project, 2093 Philadelphia Pike #1054, Claymont, DE 19703.



